Efficiency of photoinduced electron transfer in mono- and di-nuclear iridium complexes: a comparative study
Abstract
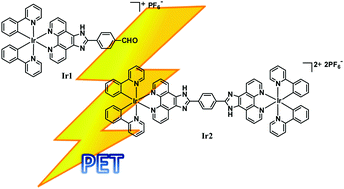
Iridium complexes have been recognized as the most widely used class of emitters because of their efficient spin–orbit coupling and hence relaxation of the spin selection rule. The strong phosphorescence of the Ir(III) complexes is induced by the triplet metal-to-ligand charge transfer (3MLCT). However, there are significant differences in the photophysical and electrochemical properties of the mono- and dinuclear Ir(III) complexes. Photoinduced energy and electron transfer (PET) is frequently observed in supramolecular Ir(III) cyclometalated complexes. Herein, we synthesized a mononuclear and a dinuclear Ir(III) complex ([Ir(ppy)2(fmp)][PF6] and [{Ir(ppy)2}2(H2bpib)][PF6]2, represented by 1 and 2 in the text, respectively) to compare their PET efficiencies. Spectroscopic and electrochemical studies reveal that 1 (consisting of a single iridium center) acts as a better electron donor as compared to 2 (consisting of two iridium centers) during PET.


 Please wait while we load your content...
Please wait while we load your content...