Organocatalytic enantioselective construction of isatin-derived N-alkoxycarbonyl 1,3-aminonaphthols via sterically encumbered hydrocarbon-substituted quinine-based squaramide†
Abstract
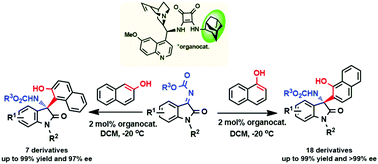
Herein, an illustrative example to synthesize chiral naphthoxazepine precursors via the aza-Friedel–Crafts reaction of N-alkoxycarbonyl isatin ketimines with naphthol using a new 2-adamantyl-substituted quinine-derived squaramide catalyst is presented; the reaction afforded the chiral-tetrasubstituted 3-amino-2-oxindoles with excellent enantioselectivity of greater than 99% ee and quantitative yields. To the best of our knowledge, this methodology is featured for being representative of the efficiency of sterically hindered hydrocarbon substituents in squaramide organocatalysts in terms of stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...