Functional polysiloxanes: a novel synthesis method and hydrophilic applications†
Abstract
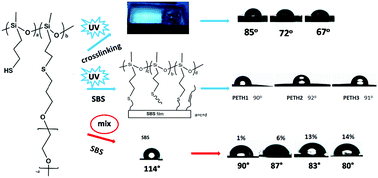
In this paper, functional dialkoxysilanes, (3-((3-chloropropyl)thio)propyl)methyldimethoxysilane, 3-((3-(dimethoxy(methyl)silyl)propyl)thio) propanoic acid, 3-methoxy-3-methyl-2,11-dioxa-7-thia-3-silatridecan-13-ol, and 3-methoxy-3-methyl-2,11,14,17,20,23,26,29,32-nonaoxa-7-thia-3-silatritriacontane, are first obtained by reacting functional alkenes with 3-(dimethoxy(methyl)silyl)propane-1-thiol in near-quantitative yields using a simple, efficient and photoinitiated thiol–ene click reaction. Then, functional polysiloxanes are synthesized from their corresponding functional dialkoxysilane monomers. This two-step method is a novel and efficient way of synthesizing functional polysiloxanes. The functional polysiloxanes show obvious fluorescence properties, which are assumed to be generated from unconventional chromophores. Furthermore, a series of copolymers (PETHs) with mercaptopropyl and polyether side chains are also obtained. They are successfully used for hydrophilic modification of a poly(styrene-b-butadiene-b-styrene) triblock copolymer. The PETH-based blue-light-emitting silicone elastomer is synthesized first via a thiol–ene click reaction, and it exhibits wonderful hydrophilicity, which may be useful in biomedical fields.


 Please wait while we load your content...
Please wait while we load your content...