Mechanism and kinetics for the reactions of methacrolein and methyl vinyl ketone with HO2 radical†
Abstract
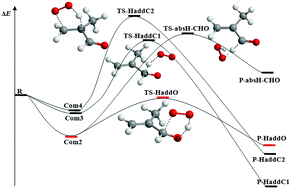
The high-level theoretical study on the reactions of methacrolein and methyl vinyl ketone with hydroperoxyl radical was carried out using the M06-2X method in conjunction with the 6-311+G(2df,2pd) basis sets. For the two reactions, all possible addition and abstraction channels initiate from the reactant complexes. The most favorable reaction channel is the concerted addition of the terminal O atom of HO2 radical to the aldehyde-C (or carbonyl-C) and H-transfer from HO2 to the aldehyde-O (or carbonyl-O) forming the α-hydroxyalkenylperoxy radical. For the methacrolein + HO2 and methyl vinyl ketone + HO2 reactions, the calculated rate constants decrease as the temperature increases from 200 K to 500 K, and at 298 K, they are 1.34 × 10−14 and 2.56 × 10−14 cm3 molecule−1 s−1, respectively. The calculated results are close to the previous theoretical and experimental observations for the reactions of HO2 radical with formaldehyde, acetaldehyde, and acetone.


 Please wait while we load your content...
Please wait while we load your content...