Highly sensitive and selective fluorescent sensor for copper(ii) based on salicylaldehyde Schiff-base derivatives with aggregation induced emission and mechanoluminescence†
Abstract
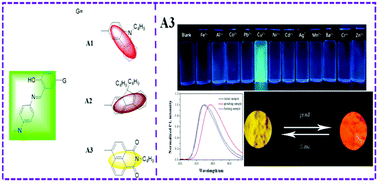
Three new Schiff-base derivatives with aggregation-induced emission (AIE) characteristics have been designed and successfully synthesized. Their photophysical properties in solution, aqueous suspension and the solid state were investigated systematically. The three compounds showed an obvious 1π–π* transition mixed with intramolecular charge transfer (ICT) properties in the UV-vis spectra, which were evidenced by spectral analysis and theoretical calculations. The compounds exhibited evident AIE characteristics with a relatively high fluorescence quantum yield in the solid state (ΦF = 15–21%). Moreover, the morphologies and sizes of the particles in water/CH3CN solutions were studied using a transmission scanning electron microscope (TEM) and dynamic light scattering (DLS). The crystallographic data for A3 suggest that the existence of intramolecular hydrogen bonding interactions restricted intramolecular rotation. Interestingly, A3 showed fluorescence turn-on sensing towards Cu2+via chelation enhanced fluorescence in a CH3CN–H2O (4 : 1, v/v) solution. It has a fast response time and a linear range of 0–20 μM for Cu2+ ions with a detection limit of 2.3 × 10−7 mol L−1. In addition, A3 also exhibits a piezofluorochromic response.


 Please wait while we load your content...
Please wait while we load your content...