Understanding the reactivity of bis(propargyl) aromatic esters towards GAP: a theoretical exploration†
Abstract
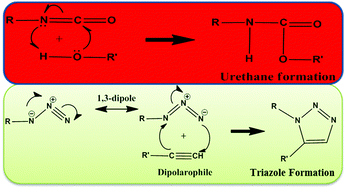
Curing is one of the essential processes of propellants to provide a matrix for the ingredients to bind together. Due to the hazardous and moisture-sensitive nature of isocyanate-based curing agents, it is necessary to replace them with non-isocyanate-based curing agents for curing the energetic binder glycidyl azide polymer (GAP). The reactivity of GAP with five non-isocyanate curing agents was studied by using reaction activation barriers and molecular electrostatic potentials (MESP). The relative trend of MESP-Vmin has predicted the relative reactivity trend of the curing agents. It has been observed that the m-bis(propargyl) aromatic ester possesses similar reactivity to the p-bis(propargyl) aromatic ester as per their negative potentials. The effect of nitro substitution has been examined for both the p- and m-bis(propargyl) aromatic esters. The nitro substitution of the p-bis(propargyl) aromatic ester lowered its reactivity as compared to the unsubstituted p-bis(propargyl) aromatic ester. The reactivity of the m-bis(propargyl) aromatic ester also reduces upon nitro substitution. However, all the studied non-isocyanate curing agents have emerged with similar reacting ability when we studied their reaction energy profiles towards curing of GAP suggesting the importance of considering the role of intermolecular interactions between bis(propargyl) ester-based curing agents and GAP which are absent in the MESP phenomenon. Further, the present work revealed the kinetic and thermodynamic superiority of the non-isocyanate curing system over the conventional hazardous isocyanate-based curing system towards the energetic binder GAP.


 Please wait while we load your content...
Please wait while we load your content...