New types of Cu and Ag clusters supported by the pyrrole-based NNN-pincer type ligand†
Abstract
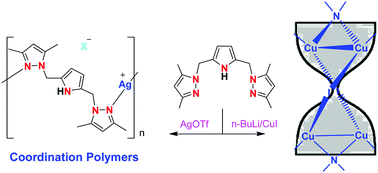
2,5-Bis(3,5-dimethylpyrazolylmethyl)pyrrole LH becomes the monoanionic NNN pincer ligand upon deprotonation of the pyrrolic NH proton. It not only gives typical pincer type complexes but also supports multinuclear complexes via its bridging mode of coordination. This was demonstrated by the synthesis and characterization of a series of copper(I) and silver(I) complexes. The reaction between LH and CuX, where X = Cl, Br and I, gave binuclear complexes of the type [Cu2(μ-X)2(μ-C4H3N-2,5-(CH2Me2pz)2-N,N)2] (1a–c). Conversely, when the reaction was carried out with the lithium salt of LH and CuI, a novel tetranuclear copper(I) complex [Cu4(μ-I)2(μ-C4H2N-2,5-(CH2Me2pz)2-N,N,N)2] 2 was obtained and its core structure resembled an ‘hourglass’. The analogous reaction with CuCl or CuBr yielded the neutral copper(II) complex [Cu(μ-C4H2N-2,5-(CH2Me2pz)2-N,N,N)2] 3 upon oxidation in air, in which L displayed the chelating bonding mode. The analogous reaction between LH and AgOTf or AgBF4 afforded the 1D coordination polymer of the type [Ag(μ-C4H3N-2,5-(CH2Me2pz)2-N,N)(X)]n where X = CF3SO3−4a or BF4−4b. Conversely, a new trinuclear silver(I) complex [Ag3(μ-C4H2N-2,5-(CH2Me2pz)2-N,N,N)3] 5 was obtained when the reaction was carried out with the lithium salt of LH and AgBF4. The structures of 1b, 1c, 3, 4a and 5 were confirmed by single crystal X-ray diffraction studies and supported by NMR, FTIR, HRMS and CHN analyses. The Cu–Cu distance of 2.6127(8) Å in 2 and the Ag–Ag distance of 2.8514(8) Å in 5 indicate the presence of d10–d10 interactions.


 Please wait while we load your content...
Please wait while we load your content...