Facile synthesis of nano-Zn/Bi–reduced graphene oxide for enhanced photocatalytic elimination of chlorinated organic pollutants under visible light†
Abstract
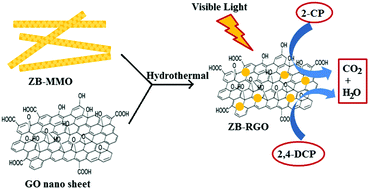
A Zn/Bi mixed metal oxide (MMO) was synthesized by a simple co-precipitation method. Then Zn/Bi-MMO was hydrothermally treated with graphene oxide (GO) to obtain a Zn/Bi–RGO co-assembly. The XRD pattern confirms the formation of highly pure Zn/Bi–RGO nanoparticles. In the co-assembled Zn/Bi–RGO hybrid, GO had converted into RGO, which was evidenced by spectroscopy techniques, such as FT-IR, Raman, XPS, etc. UV-Vis DRS spectra infer that the prepared hybrid has significant absorption in the visible light region. The photocatalytic studies were conducted using 2-chlorophenol (2-CP) and 2,4-dichlorophenol (2,4-DCP) and the hybrid offered almost complete degradation of pollutants as compared to pure Zn/Bi-MMO. The enhanced photocatalytic activity of Zn/Bi–RGO hybrids is due to the homogeneous distribution of Zn/Bi-MMO over RGO sheets as well as electron transportation efficacy of the RGO sheets. The mechanism of catalysis is expected to have two key events; namely a charge transfer mechanism, which initiates the reaction, followed by an electron–hole aided radical mechanism.


 Please wait while we load your content...
Please wait while we load your content...