New sulfonated copoly(triazole imide)s synthesized by a click chemistry reaction with improved oxidative stability
Abstract
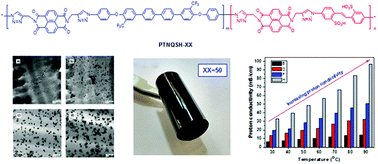
Herein, novel soluble sulfonated copoly(triazole imide)s with different ion exchange capacities were synthesized by a click reaction. The structures of the resulting copoly(triazole imide)s were characterized by FTIR and 1H NMR spectroscopic techniques. These copoly(triazole imide)s were soluble in different organic solvents and formed flexible films with good mechanical properties along with high thermal stabilities. The presence of triazole and flexible moieties contributed towards improving the oxidative stability and swelling properties. The proton conductivities of the copolymers were found to be in the range of 15–98 mS cm−1 at 90 °C in water. Transmission electron microscopy was used to observe the microphase separation of the ionic and hydrophobic domains of the copolymers.


 Please wait while we load your content...
Please wait while we load your content...