Syntheses and characterization of monobasic tridentate Cu(ii) Schiff-base complexes for efficient oxidation of 3,5-di-tert-butylcatechol and oxidative bromination of organic substrates†
Abstract
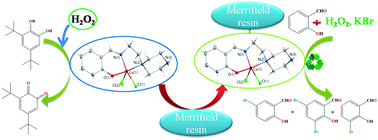
Two monomeric copper complexes [CuII(sal-ppzH)Cl2] (1) and [CuII(hyap-ppzH)Cl2] (2) were synthesized by reacting CuCl2·2H2O with the monobasic tridentate Schiff-base ligands [Hsal-ppz] (I) and [Hyap-ppz] (II) (derived by reacting 1-(2-aminoethyl) piperazine with salicylaldehyde and 2-hydroxyacetophenone) respectively. Elemental analysis, IR, UV-Vis, 1H NMR and 13C NMR data confirm the structures of the ligands and those of the complexes. Both complexes are monomeric in nature in the solid state and in solution as well. Single crystal XRD data suggest a distorted square pyramidal geometry for 1 crystallized in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. DFT studies established a similar molecular structure for 2. The synthesized metal complexes [CuII(sal-ppzH)Cl2] (1) and [CuII(hyap-ppzH)Cl2] (2) successfully catalyzed the oxidation of 3,5-di-tert-butylcatechol (3,5-DTBC) in methanol in the presence of H2O2 with high Kcat values of 1.182 × 104 mmol h−1 and 2.880 × 104 mmol h−1, respectively. [CuII(sal-ppzH)Cl2] (1) and [CuII(hyap-ppzH)Cl2] (2) were also anchored into the polymeric matrix of chloromethylated polystyrene and were analyzed by TGA, atomic absorption spectroscopy (AAS), EPR, scanning electron microscopy (SEM) as well as energy dispersive X-ray (EDX) analysis. The polymer grafted metal complexes were used as catalyst precursors in the oxidative bromination of salicylaldehyde in the presence of H2O2, KBr and HClO4. Under optimized reaction conditions, both catalysts show nearly quantitative oxidative bromination of salicylaldehyde, with the order of % products formed being 5-bromosalicylaldehyde > 3,5-dibromosalicylaldehyde > 3-bromosalicylaldehyde. Plausible reactive species involved in the catalytic cycle are identified by UV-Vis spectroscopy, pH metric titration, ESI-MS, EPR and DFT studies.
space group. DFT studies established a similar molecular structure for 2. The synthesized metal complexes [CuII(sal-ppzH)Cl2] (1) and [CuII(hyap-ppzH)Cl2] (2) successfully catalyzed the oxidation of 3,5-di-tert-butylcatechol (3,5-DTBC) in methanol in the presence of H2O2 with high Kcat values of 1.182 × 104 mmol h−1 and 2.880 × 104 mmol h−1, respectively. [CuII(sal-ppzH)Cl2] (1) and [CuII(hyap-ppzH)Cl2] (2) were also anchored into the polymeric matrix of chloromethylated polystyrene and were analyzed by TGA, atomic absorption spectroscopy (AAS), EPR, scanning electron microscopy (SEM) as well as energy dispersive X-ray (EDX) analysis. The polymer grafted metal complexes were used as catalyst precursors in the oxidative bromination of salicylaldehyde in the presence of H2O2, KBr and HClO4. Under optimized reaction conditions, both catalysts show nearly quantitative oxidative bromination of salicylaldehyde, with the order of % products formed being 5-bromosalicylaldehyde > 3,5-dibromosalicylaldehyde > 3-bromosalicylaldehyde. Plausible reactive species involved in the catalytic cycle are identified by UV-Vis spectroscopy, pH metric titration, ESI-MS, EPR and DFT studies.


 Please wait while we load your content...
Please wait while we load your content...