Complexation behaviour of caffeic, ferulic and p-coumaric acids towards aluminium cations: a combined experimental and theoretical approach†
Abstract
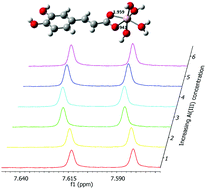
Here we have studied the complexation of caffeic, ferulic and p-coumaric acids with Al(III) under physiological conditions (i.e. at 37 °C and in 0.16 M NaCl). Solubility and protonation constants of these hydroxycinnamic acids were firstly determined in order to evaluate the competition of the ligands for the Al(III) and H+ ions. Speciation analysis made by potentiometric titrations and supported by UV and 1H-NMR data shows that a pH-dependent complexation occurs at 1 : 1 and 2 : 1 ligand-to-Al(III) ratios. Notably, these data combined with computational (DFT) results unequivocally indicate that the complexation site is the carboxylate group of the ligands thus excluding any involvement of the phenolic sites.


 Please wait while we load your content...
Please wait while we load your content...