Aqua coordination to attenuate the luminescence properties of europium(iii)–phosphine oxide porous coordination polymers†
Abstract
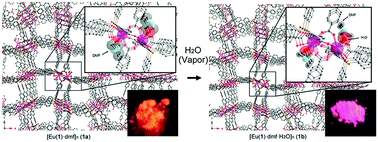
Europium(III)–phosphine oxide porous coordination polymers (Eu–PCPs) constructed from Eu(OTf)3 and tris(4-(4′-carboxylphenyl)phenyl)phosphine oxide as ligands are reported. X-Ray diffraction data of Eu–PCPs are collected at 273 K with synchrotron radiation. The europium complex is shown to crystallize in the centrosymmetric orthorhombic space group Pcca. The Eu atom is surrounded by eight neighboring oxygen atoms from six ligands and a coordinated DMF molecule. Furthermore, the two Eu centers are quadruply bridged by four carboxylates. They exhibit an infinite three-dimensional structure that incorporates an open pore structure, which is thermally robust and has an N2 Brunauer–Emmett–Teller surface area of 58.5 m2 g−1 and a micropore volume of 0.756 cm3 g−1. Excitation of the materials led to five sharp emission peaks arising from the 5D0 → 7FJ transitions (J = 0, 1, 2, 3 and 4). The absolute quantum yield of Eu–PCPs is determined as 22% at room temperature. In addition, exposure of single crystals of Eu–PCPs to H2O vapor causes attenuation of the luminescence intensity. This process involves a single-crystal-to-single-crystal transformation. In the crystal structures of the Eu–PCPs, the ligand exchange from DMF to H2O, which acts to quench the lanthanide luminescence, is directly observed.


 Please wait while we load your content...
Please wait while we load your content...