Enantioselective synthesis of dihydroquinazolinone derivatives catalyzed by a chiral organocatalyst†
Abstract
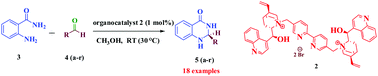
The asymmetric condensation/amine addition cascade sequence of 2-aminobenzamide and aldehydes, catalyzed by a novel chiral organocatalyst, was realized. The organocatalyst was found to be very effective and highly enantioselective for such cascade reactions at room temperature, affording 2,3-dihydroquinazolinones in excellent yields (up to 99%) with enantiomeric excesses of up to 97%. The best level of stereocontrol was obtained for aromatic/aliphatic aldehydes with ortho, para or meta substituents.


 Please wait while we load your content...
Please wait while we load your content...