Addition of N-nucleophiles to gold(iii)-bound isocyanides leading to short-lived gold(iii) acyclic diaminocarbene complexes†
Abstract
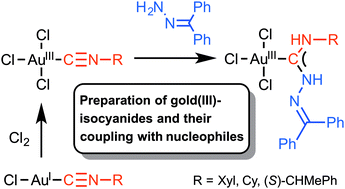
Reaction of [AuCl3(CNR1)] (R1 = Xyl, Cy, (S)-CHMePh) with amines unexpectedly proceeds via the redox pathway giving gold(I)–isocyanides and imines, while the addition of benzophenone hydrazone to the isocyanide ligand in [AuCl3(CNR1)] at RT leads to short-lived gold(III) acyclic diaminocarbene complexes [AuCl3{C(NHNCPh2)NHR1}].


 Please wait while we load your content...
Please wait while we load your content...