PEG-600 mediated one-pot reaction of 3-acetyl-2H-chromen-2-one with heterylthiols and phenylthioureas using tetrabutylammonium tribromide as an efficient green reagent†
Abstract
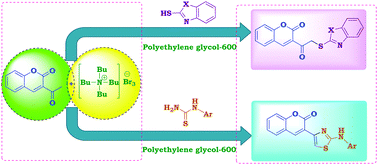
A simple method for the one-pot reaction of 3-acetyl-2H-chromen-2-one with different heterylthiols and phenylthioureas under green conditions using tetrabutylammonium tribromide (TBATB) as an efficient reagent has been described. 3-Acetyl-2H-chromen-2-one was reacted with TBATB in acetic acid to give 3-(2-bromoacetyl)-2H-chromen-2-one. Poly(ethylene glycol) 600 (PEG-600) was found to be the effective solvent for this reaction. Thus, a one-pot reaction of equimolar amounts of 3-acetyl-2H-chromen-2-one, TBATB and heterylthiols or phenylthioureas, resulted in the formation of heterylthioacetylcoumarins or coumarinylthiazoles, respectively. The effect of the solvent on these reactions was studied. The merits of this preparation are the mild reaction conditions, easy workup, good yields and use of PEG-600 as a green reaction medium.


 Please wait while we load your content...
Please wait while we load your content...