Selective hydration of asymmetric internal aryl alkynes without directing groups to α-aryl ketones over Cu-based catalyst†
Abstract
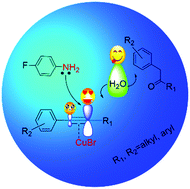
Hydration of internal aryl alkynes to provide aryl carbonyl compounds is a class of important reactions and has been widely investigated. However, the hydration of asymmetric internal aryl alkynes without directing groups usually gives an aryl ketone or a mixture of aryl ketone and α-aryl ketone. High regioselectivity to α-aryl ketone is a great challenge and has not been reported. Herein, we found that CuBr and p-fluoroaniline had an excellent synergistic effect in catalyzing the hydration of internal aryl alkynes without directing groups to α-aryl ketones with regioselectivity up to more than 90%, which is much greater than those reported. The reaction mechanism was proposed, and the reason for the high selectivity was clarified by a combination of density functional theory (DFT) calculation, condensed dual descriptor (CDD) study, and experimental results. It was demonstrated that the formations of α-aryl ketone and aryl ketone were promoted by different catalytic active species, CuBr and CuBr[p-fluoroaniline], respectively. CuBr enlarged the difference of electron population on the two triple-bond carbon atoms, resulting in α-aryl ketones as the main products.


 Please wait while we load your content...
Please wait while we load your content...