Nuclearity dependent solvent contribution to the catechol oxidase activity of novel copper(ii) complexes derived from Mannich-base ligand platforms: synthesis, crystal structure and mechanism†
Abstract
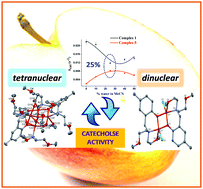
A set of tetra- and dinuclear copper(II) complexes, [Cu4(L1)(μ-O)(OAc)4] (1), [Cu2(L2)2](ClO4)2 (2), [Cu2(L2)2(OAc)2] (3), [Cu2(L2)2(Br)2] (4) and [Cu2(L2)2(Cl)2] (5), were synthesized from two Mannich-base ligands HL1 and HL2, where HL1 = 2,6-bis[bis(2-methoxyethyl)aminomethyl]-4-methylphenol and HL2 = 2-[bis(2-methoxyethyl)aminomethyl]-4-methylphenol. The catalytic efficiency of the complexes for catecholase activity was systematically evaluated by spectrophotometry using 3,5-di-tert-butylcatechol (3,5-DTBC) and tetrachlorocatechol (TCC) in pure dry acetonitrile (MeCN) and water–MeCN solvent mixtures (10%, 25%, 40% and 50% water). In spite of remarkable rate enhancements by the tetranuclear species in MeCN (kcat = 0.0218 s−1), complex 1 follows a reverse trend in the solvent dependent kinetic study compared to complexes 2–5 with a minimum at 25% water (kcat = 0.0118 s−1) under similar experimental conditions. Theoretical modeling involving the reactions of complexes 1, 3 and 5 provides the counterintuitive rationalization of the apparently dramatic behaviour of these novel catechol models on addition of water to the MeCN solution. More importantly, it vindicates the combination of the contradictory parameters like alcoholysis and hydrolysis as the governing factors for kcat extrema in catalytic pathways. Thus, the reactivity and mechanism of Mannich-base metallocatalysts across water-induced oxidation of catechols is predictable through their nuclearity.


 Please wait while we load your content...
Please wait while we load your content...