N-Insertion reaction mechanisms of phenyl azides with a hafnium hydride complex: a quantum chemistry calculation†
Abstract
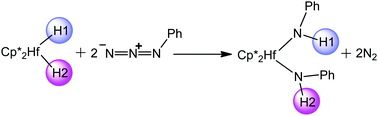
Density functional theory (DFT) calculations were performed to investigate the detailed mechanisms for the N-insertion reaction of phenyl azides with a hafnium hydride complex. This reaction involves an intermolecular hydride transfer from the hafnium center of complex 1 Cp2*HfH2 to the terminal nitrogen atom of a phenyl azide. Subsequently, a 1,3 hydrogen shift from the N1 atom to the N3 atom takes place, accompanied by cleavage of the N2–N3 bond to provide amido complex 3 Cp2*HfH(NHPh) and dinitrogen. A further reaction is related to the intermolecular hydride transfer from the hafnium center to the N1′ atom of a second phenyl azide, followed by the formation of the final product, bis(amido) complex 9 Cp2*HfH(NHPh)2via the liberation of the second dinitrogen, which is the rate-determining step with an overall barrier of 29.8 kcal mol−1. Frontier molecular orbital theory analysis shows that phenyl azides are activated by nucleophilic attack by the hydride ligand, which is consistent with our previous studies of N2O activation by other transition-metal hydride complexes.


 Please wait while we load your content...
Please wait while we load your content...