Binuclear chromium carbonyl complexes of methylaminobis(difluorophosphine): metal–metal bonds versus four-electron donor bridging carbonyl groups†
Abstract
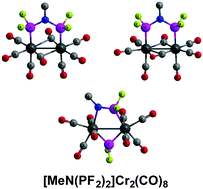
Binuclear chromium carbonyl complexes of the general type [MeN(PF2)2]mCr2(CO)n, including the experimentally known [MeN(PF2)2]3Cr2(CO)n (n = 6, 5) species, have been studied by density functional theory (DFT) methods. The lowest energy structures for the three series of [MeN(PF2)2]mCr2(CO)n (m = 1, 2, 3) structures can be grouped into three triads, namely [MeN(PF2)2]Cr2(CO)n (n = 10, 9, 8), [MeN(PF2)2]2Cr2(CO)n (n = 8, 7, 6), and [MeN(PF2)2]3Cr2(CO)n (n = 6, 5, 4). The carbonyl richest structures of each triad, namely [MeN(PF2)2]Cr2(CO)10, [MeN(PF2)2]2Cr2(CO)8, and [MeN(PF2)2]3Cr2(CO)6 have all terminal carbonyl groups, no chromium–chromium bond, and the MeN(PF2)2 ligands bridging the pair of chromium atoms. However, for [MeN(PF2)2]3Cr2(CO)6 a structure with two of the three MeN(PF2)2 ligands chelating to single chromium atoms are energetically competitive. Low-energy singlet spin state structures for the intermediate and carbonyl poorest members of each triad can incorporate a variety of features such as chromium–chromium single and double bonds, MeN(PF2)2 ligands split into bridging MeNPF2 + PF2 groups, and four-electron donor bridging η2-μ-CO groups as required to give each chromium atom the favored 18-electron configuration. Such four-electron donor bridging η2-μ-CO groups are not found in low-energy structures of related binuclear carbonyl complexes [MeN(PF2)2]mM2(CO)n (M = Fe, Ni; m = 1, 2) of the later transition metals iron and nickel.


 Please wait while we load your content...
Please wait while we load your content...