Boronate-affinity hollow molecularly imprinted polymers for the selective extraction of nucleosides
Abstract
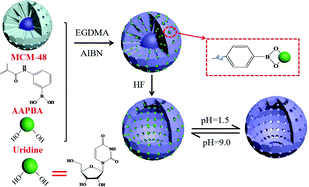
Boronate-affinity hollow molecularly imprinted polymers (h-MIPs) were prepared and applied to the selective enrichment of four nucleosides in traditional Chinese medicine injection samples, followed by HPLC detection. The synthesis mechanism of the h-MIPs was studied. Due to a large amount of boronic acid groups were introduced by 3-aminophenylboronic acid (APBA) in the outermost polymer shell of the MIPs, which was of great importance for improving the enrichment efficiency of the nucleosides. Furthermore, owing to the hollow structure of the h-MIPs, more binding cavities were located on the inner and outer surfaces of the h-MIPs, which could facilitate the removal of template molecules from the polymers and the rebinding of the target molecules to the polymers. Under optimal conditions, the detection limits of the four nucleosides were in the range of 0.012–0.033 μg mL−1. At the different spiked levels, the recoveries of the nucleosides were in the range of 63.8–108.9%. The proposed method was successfully applied to determine four nucleosides in five injection samples. The boronate-affinity h-MIPs provided a promising selective enrichment platform for precise nucleoside analysis.


 Please wait while we load your content...
Please wait while we load your content...