Pyrene–fluorescein-based colour-tunable AIE-active hybrid fluorophore material for potential live cell imaging applications†
Abstract
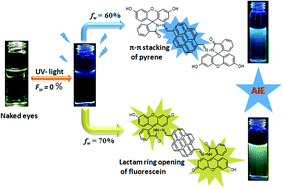
A novel hybrid fluorophore (FHPY) has been synthesized based on a condensation reaction of two standard fluorescent hydrophobic–hydrophilic molecules, viz. pyrene and fluorescein, with an objective to tune the aggregation-induced emission (AIE) along with the morphology. Owing to the distinct photophysical properties of pyrene and fluorescein, the hybrid FHPY dramatically exhibits a fluorescence change from colourless to yellow-green via a blue colour upon varying the volume fraction of water (poor solvent) in methanol (good solvent). FHPY has exhibited not only AIE, but also an outstanding quantum yield (ΦF) of 97% at a 70% water fraction in methanol (70 : 30, v/v). We attribute the reason behind the tuning of the AIE and quantum yield to the opening of the lactam ring of fluorescein as well as to the amassing of hydrophobic pyrene at a certain water fraction. The mechanism involved in the AIE has been well supported by detailed UV-vis, fluorescence, lifetime, SEM, AFM, DFT, PXRD and 1H NMR experiments. In addition, FHPY serves as a good candidate for the live cell imaging of HeLa cells.


 Please wait while we load your content...
Please wait while we load your content...