A new and versatile one-pot strategy to synthesize alpha-bromoketones from secondary alcohols using ammonium bromide and oxone†
Abstract
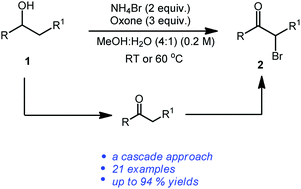
A new, efficient and green protocol for the one-pot synthesis of α-bromoketones from secondary alcohols using cheap, air stable and non-toxic reagents such as NH4Br and oxone has been developed. This reaction proceeds via two consecutive steps such as oxidation of secondary alcohols and oxidative bromination of in situ generated ketones.


 Please wait while we load your content...
Please wait while we load your content...