Development of kinetic model for CO hydrogenation reaction over supported Fe–Co–Mn catalyst
Abstract
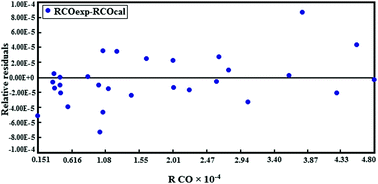
Kinetic modeling of CO hydrogenation over Fe–Co–Mn catalyst was investigated; a ternary catalyst was prepared using incipient wetness impregnation. The kinetic parameters were determined from experiments carried out in a fixed bed micro-reactor under the following process conditions: T = 523.15–533.15 K, P = 1–10 bar, H2/CO = 1/1–3/1 and space velocity = 4500 h−1. Seventeen rate expressions were derived from five different mechanisms according to Langmuir–Hinshelwood–Hougen–Watson (LHHW) and Eley–Rideal (ER) mechanisms and were tested against the experimental data. Non-linear regression method was used to estimate different kinetic parameters; according to the obtained experimental results and using statistical criteria, one kinetic expression based on LHHW mechanism (−rCO = KPCO·(PH2)0.5/[1 + αPCO + (βPH2)0.5]2) was chosen as the best fitted model. The activation energy for this model was found to be 84.5 kJ mol−1. The physicochemical properties of the catalyst were investigated using XRD, SEM, BET and TPR techniques.


 Please wait while we load your content...
Please wait while we load your content...