Tuning the fluorescence emission in mononuclear heteroleptic trigonal silver(i) complexes†
Abstract
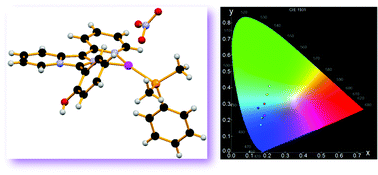
Seven mononuclear heteroleptic silver(I) complexes, [Ag(N∩N)(PR3)](NO3) (N∩N = 2-(1-(pyridin-2-yl)imidazo[1,5-a]pyridin-3-yl)phenol; P = PPh3 (1), PMe2Ph (2), PMePh2 (3), P(p-tolyl)3 (4), P(nBu)3 (5), P(OPh)3 (6), and P(OEt)3 (7)), have been prepared and characterized. In these compounds, N∩N acts as a bidentate ligand with the pyridine ring and the pyridine-like nitrogen of the imidazo[1,5-a]pyridine group. The coordination around silver is completed by the monodentate phosphine, the nitrate anion being not bound to the metal, which then displays a trigonal planar geometry as confirmed by the X-ray crystal structure of compounds 1, 2, 5 and 6. 31P{1H} NMR and conductivity measurements were performed to settle on the behavior of these complexes in solution. The photophysical properties of these species have been investigated, both in solution and in the solid state: they showed intense fluorescence when excited with UV light, with λmax of emission comprised between 440–460 nm (in solution) and 460–498 nm (solid state) and lifetime decay of a few nanoseconds. In the solid state, a reasonable tuning of the emission is observed according to the electronic features of the coordinated phosphine.


 Please wait while we load your content...
Please wait while we load your content...