Croconato-bridged copper(ii) complexes: synthesis, structure and magnetic characterization†
Abstract
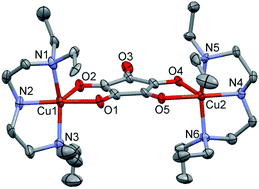
Three croconato-bridged Cu(II) complexes were synthesized and structurally characterized including dinuclear [Cu2(Et4dien)2(μ1,2,3,4-C5O5)](ClO4)2·3H2O (1) and [Cu2(pmap)2(μ1,2,3,4-C5O5)](ClO4)2·2H2O (2) and the 1D coordination polymer {[Cu(dmpzp)(μ1,2,3,4-C5O5)]·H2O}n (3), where Et4dien = N,N′,N′′,N′′-tetraethylethylenetriamine, pmap = bis(2-(2-pyridyl)ethyl)-(2-pyridylmethyl)amine and dmpzp = 1-(2-methylpyridyl)-3,5-dimethyl-1-H-pyrazole. The synthesized complexes were further characterized by a single crystal X-ray analysis which revealed the bis-bidentate chelating nature of the μ1,2,3,4-croconato ligand with the intra-dimer Cu⋯Cu bond distances ranging from 6.79 to 7.69 Å across the bridged croconate. The magnetic susceptibility measurements at varying temperatures (1.9–300 K) showed that the complexes exhibit a weak antiferromagnetic (AF) coupling with J values = −2.67 cm−1 for 1 and 2, and −4.05 cm−1 for 3. The weak AF coupling was also predicted based on the DFT calculations. The magnetic properties of the complexes are discussed in light of their structures and compared with the previously reported croconato-bridged Cu(II) complexes.


 Please wait while we load your content...
Please wait while we load your content...