New Ru(ii) N′NN′-type pincer complexes: synthesis, characterization and the catalytic hydrogenation of CO2 or bicarbonates to formate salts†
Abstract
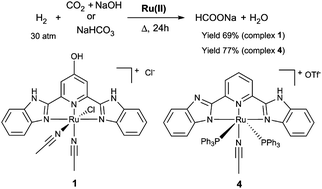
[RuCl(L1)(MeCN)2]Cl (1) and [RuCl(L2)(MeCN)2]Cl (2) complexes were prepared through the reaction of [RuCl2(p-cymene)]2 with 2,6-bis(benzimidazole-2-yl)-4-hydroxy-pyridine (L1) or 2,6-bis(benzimidazole-2-yl) pyridine (L2) in acetonitrile, respectively. The treatment of [Ru(OTf)(L2)(MeCN)2]OTf (3) with 1 equivalent of PPh3 in ethanol resulted in the formation of [Ru(L2−1)(MeCN)(PPh3)2]OTf (4), in which one of the N–H moieties of L2 is deprotonated to give an anionic ligand (L2−1). It was found that complex 1 can catalyze the hydrogenation of CO2 to formate salts, producing sodium formate in 34.0% yield with a turnover number (TON) of 407 under the optimized conditions. Further investigations revealed that complexes 1–4 can efficiently catalyze the hydrogenation of sodium bicarbonate to sodium formate, and the catalytic activity follows the order 4 > 1 > 2 ≈ 3. In particular, sodium formate was obtained in good yield (77%) with a high TON (1530) when complex 4 was used as the catalyst. The present results illustrate a new example of Ru(II) complexes bearing a rigid N′NN′ framework for the efficient hydrogenation of CO2 to formate salts in a homogeneous system.


 Please wait while we load your content...
Please wait while we load your content...