A green and efficient photo-driven route for the selective oxidation of aqueous isopropanol solution to pinacol (C6) with hydrogen peroxide
Abstract
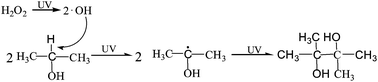
Selective oxidation of alcohols to highly valued chemicals in a clean manner (e.g., with water as a solvent and O2 or H2O2 as the primary oxidant) is the subject of considerable research attention. This paper presents a simple and environmentally friendly approach for the selective oxidation of isopropanol to pinacol C6 (2,3-dimethyl-2,3-butanediol) in water with hydrogen peroxide (H2O2) through UV irradiation under mild conditions. A selectivity of 82–95% is achieved at H2O2 feeding rates below 4 mL h−1. The introduction of TiO2 nanotubes can further accelerate isopropanol conversion and enhance pinacol C6 formation. The highest formation rate of pinacol C6 and the highest conversion rate of isopropanol are 29.4 and 71.4 mmol h−1, respectively. The proposed strategy provides a green solution for the synthesis of aliphatic pinacol and offers a new design for the construction of C–C bonds with alphatic alcohol.


 Please wait while we load your content...
Please wait while we load your content...