Bolaamphiphilic liquid crystals based on bis-imidazolium cations†
Abstract
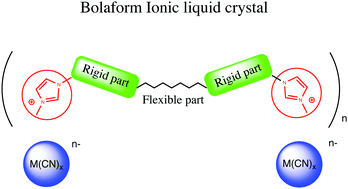
Syntheses are reported for new bolaform ionic liquids 2a–f containing the cyanometallate anions [Ag(CN)2]− (2a), [Ni(CN)4]2− (2b), [Pt(CN)4]2− (2c), [Co(CN)6]3− (2d), [Fe(CN)6]3− (2e) and [Fe(CN)6]4− (2f), respectively, associated with a bis(imidazolium) dication. Except for those containing the square-planar nickelate and platinite anions, all the compounds displayed thermotropic behaviour, exhibiting lamellar smectic A phases. The mesomorphic behavior and phase transition temperatures were investigated by polarizing optical microscopy (POM), differential scanning calorimetry (DSC) and small-angle X-ray scattering (SAXS). To investigate the nature anion influence on its interactions with the cation, crystal structures from single crystal X-ray diffraction have been obtained for both [Ag(CN)2]− and [Ni(CN)4]2− derivatives. These studies showed that the square planar Ni anion disrupts the lateral cohesion of the ionic layers. This may explain the loss of liquid crystal behavior in this case and Pt salt.


 Please wait while we load your content...
Please wait while we load your content...