The basket-type dimer layers based on tetra-electron reduced heteropoly blue directed by copper/nickel and strontium linkers†
Abstract
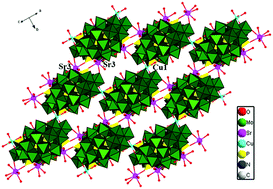
The Sr(II) and Cu(II)/Ni(II) cations are introduced as linkers into basket POM systems, leading to two isostructural 2-D assemblies (H3bth)4[{Sr0.5(H2O)0.5(H2O)}2{Sr(H2O)4}2{Cu0.5(H2O)}2{Sr⊂P6MoV4MoVI14O73}2]·5H2O (1) and (H3bth)4[{Sr0.5(H2O)0.5(H2O)}2{Sr(H2O)3(H2O)0.5(H2O)0.25}2{Ni0.5(H2O)}2{Sr⊂P6MoV4MoVI14O73}2]·3H2O (2) (bth = 1,6-bis(triazole)hexane), which have been characterized by X-ray crystallographic analyses, FT-IR, UV-Vis, TG-DTA, XRD, XPS, BET, and elemental analyses. The compounds are based on four-electron reduced heteropoly blue {Sr⊂P6MoV4MoVI14O73}, which is made up of the tetra-vacant γ-Dawson cluster, {P2Mo14} and the handle-shaped {P4Mo4} segment encapsulated a Sr(II) ion in the central cave. The compounds exhibit isostructural 2-D dimeric layers linked by Cu(II)/Ni(II) and Sr(II) linkers, which are observed for the first time as 2-D dimeric layer assemblies based on a basket-type cluster. The compounds exhibit highly efficient catalytic ability for the degradation of typical dyes under UV irradiation and bifunctional electrocatalytic behaviors for oxidation of dopamine (DA) and reduction of NO2−.


 Please wait while we load your content...
Please wait while we load your content...