An ionic liquid promoted approach to bitriazolyl compounds as succinate–ubiquinone oxidoreductase inhibitors
Abstract
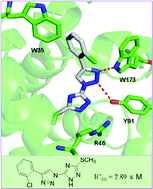
The respiratory chain succinate–ubiquinone oxidoreductase (SQR or complex II) is a promising target for fungicide discovery. As a continuation of our research work on the development of new fungicides, a series of bitriazolyl compounds were designed and synthesized in excellent yields by an ionic liquid promoted 1,3-dipolar Huisgen cycloaddition reaction of azides and akynes. These newly synthesized compounds were characterized by 1H NMR, 13C NMR and HR-MS spectroscopy. The in vitro assay indicated that several compounds displayed good inhibitory effects against porcine succinate–cyctochrome reductase (SCR) with IC50 values ranging from 2.89 to 61.19 μM. Compound 1b with an IC50 value of 2.89 μM, comparable to the commercial control penthiopyrad, was identified as the most promising inhibitor. Further evaluation of the representative compounds against respective SQR and cyt bc1 indicated that their inhibitory potency against SQR was much higher than that against cyt bc1, suggesting that SQR might be a potential target of these inhibitors. Furthermore, molecular docking studies suggested that strong hydrogen bonding and π–π stacking interactions might be responsible for a higher SQR inhibitory effect of compound 1b as compared to that of compounds 1d and 2b. Consequently, bitriazolyl compounds, a totally new skeleton that is distinct from the existing commercial SQR-inhibiting fungicides, were discovered, which could potentially be a new lead for further development of SQR inhibitors.

 Please wait while we load your content...
Please wait while we load your content...