“Host–guest” binding of a luminescent dinuclear Au(i) complex based on cyclic diphosphine with organic substrates as a reason for luminescence tuneability†
Abstract
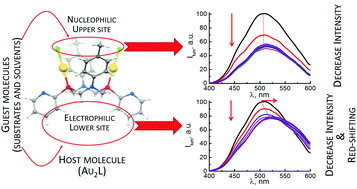
This work introduces a luminescent dinuclear Au(I) complex with a cyclic PNNP ligand ((AuCl)2L) as a “host” molecule with two binding sites, “upper” and “lower”. The “upper” binding site is nucleophilic due to two preorganized Au–Cl moieties, while the “lower” one is electrophilic due to positive partial charges of hydrogen atoms of P–CH2–N moieties. The “host–guest” binding is a reason for both solvent- and substrate-induced tuning of the complex luminescence. Organic cations, namely N-methylpyridinium and trimethylammonium, are revealed as substrates able to bind via the “upper” site of the complex. Acetone, diphenylketone, DMSO, DMF and acetonitrile exemplify substrates able to bind with both the binding sites of the complex. The binding via “lower” sites leads to changes in mutual arrangement of pyridyl moieties and P–Au bonds of the complex, which results in a more pronounced effect on the excited state energy relative to the binding via the “upper” site. Substrate-induced tuning of the luminescence is affected by the nature of the solvent due to competitive “host–guest” binding of (AuCl)2L with solvent molecules.

 Please wait while we load your content...
Please wait while we load your content...