A thermally activated manganese 1,4-benzenedicarboxylate metal organic framework with high anodic capability for Li-ion batteries†
Abstract
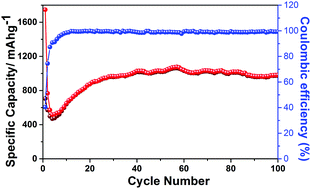
Metal organic frameworks (MOFs) with considerable structural versatility are considered to be potential materials for energy storage. In this work, a Mn-1,4-benzenedicarboxylate (Mn-1,4-BDC) MOF was synthesized by reaction of 1,4-benzenedicarboxylic acid (1,4-BDC) with manganese(II) chloride (MnCl2) using a solvothermal method. When applied as an anode for lithium-ion batteries, the activated Mn-1,4-BDC@200 electrode delivered a high reversible lithium storage capacity of 974 mA h g−1 after 100 cycles at a current density of 100 mA g−1, exhibiting one of the best lithium storage properties among the reported metal organic frameworks (MOFs), also known as coordination polymer (CP) anodes. The excellent electrochemical performance of the Mn-1,4-BDC electrode is also comparable with those reported for Mn2O3 and Mn3O4 nanostructures calcined from Mn-based MOF templates.


 Please wait while we load your content...
Please wait while we load your content...