Photophysical comparisons of PEGylated porphyrins, chlorins and bacteriochlorins in water†
Abstract
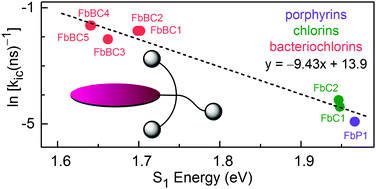
Comparison of the photophysics of analogous members of the porphyrin, chlorin, and bacteriochlorin family has rarely been carried out, and most studies of individual members have been performed in non-aqueous media. Advances in synthesis and molecular design now enable such comparative studies in aqueous solution. A water-soluble bioconjugatable free base porphyrin (FbP1) has been synthesized, complementing recently prepared chlorin and bacteriochlorin analogues. The porphyrin (a trans-AB architecture; i.e., 5,15-substitution pattern) is equipped with one meso-aryl group that bears three short polyethylene glycol (PEG) substituents at the 2,4,6-positions for aqueous solubility, and one meso-aryl group that bears an oxyacetic acid group at the p-position for bioconjugation. Spectroscopic examination of porphyrin FbP1 gave the following quantum yields in DMF versus water: fluorescence (Φf = 0.077 vs. 0.066), intersystem crossing (Φisc = 0.90 vs. 0.84), and internal conversion (Φic = 0.023 vs. 0.094). The increase in Φic upon moving from DMF into water reflects the 4-fold increase in rate constant for internal conversion, from (600 ns)−1 to (140 ns)−1. Photophysical comparisons (τS, kf, kic, kisc, Φf, Φic, Φisc) were made with analogous chlorins and bacteriochlorins in DMF and water. The value of kic in both media increases in the order (visible-absorbing) porphyrin < (red-absorbing) chlorin < (near-infrared-absorbing) bacteriochlorin, consistent with the general S1-energy dependence of kic expected on the basis of the energy-gap law for non-radiative decay. In summary, there are now satisfactory molecular designs, viable synthetic routes, and thoroughly characterized photophysical properties that permit aqueous solution studies of a complement of porphyrins, chlorins, and bacteriochlorins.

 Please wait while we load your content...
Please wait while we load your content...