Luminescent CuI thiocyanate complexes based on tris(2-pyridyl)phosphine and its oxide: from mono-, di- and trinuclear species to coordination polymers†
Abstract
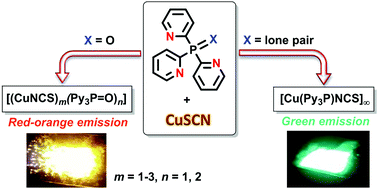
Tris(2-pyridyl)phosphine oxide reacts with CuSCN to form a variety of luminescent complexes, depending on the specified metal-to-ligand ratio and the solvent used, viz. mononuclear [Cu(N,N′,N′′-Py3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(NCS)], dinuclear (N,N′-Py3P
O)(NCS)], dinuclear (N,N′-Py3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Cu(SCNNCS)Cu[(N,N′-Py3P
O)Cu(SCNNCS)Cu[(N,N′-Py3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)], their co-crystal (2 : 1, correspondingly) and trinuclear {Cu(NCS)[SCNCu(N,N′,N′′-Py3P
O)], their co-crystal (2 : 1, correspondingly) and trinuclear {Cu(NCS)[SCNCu(N,N′,N′′-Py3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)]2}. In the solid state, these complexes feature red-orange emission upon UV photoexcitation. The reaction of tris(2-pyridyl)phosphine with CuSCN quantitatively produces an almost insoluble coordination polymer, [Cu(Py3P)NCS]n, which exhibits bright green emission. The synthesized compounds are the first members of the hitherto unknown family of Cu(I) thiocyanate complexes supported by tripodal ligands.
O)]2}. In the solid state, these complexes feature red-orange emission upon UV photoexcitation. The reaction of tris(2-pyridyl)phosphine with CuSCN quantitatively produces an almost insoluble coordination polymer, [Cu(Py3P)NCS]n, which exhibits bright green emission. The synthesized compounds are the first members of the hitherto unknown family of Cu(I) thiocyanate complexes supported by tripodal ligands.


 Please wait while we load your content...
Please wait while we load your content...