Can a Diels–Alder reaction be accelerated in a supersaturated solvent at room temperature?†
Abstract
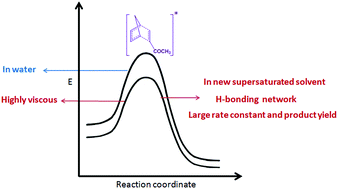
A new supersaturated solvent is proposed to accelerate the Diels–Alder reaction between cyclopentadiene and methyl acrylate by several times. The proposed supersaturated solvent consists of various carbohydrates, organic acid and organic ketone in water, beyond their solubility limits in water, at room temperature. The results demonstrate that the presence of excess –OH groups from the carbohydrates plays a pivotal role leading to the increased reactivity and selectivity of the Diels–Alder reaction. The role of hydrogen bond accelerators and polarity of the solvent have been analyzed through a comparative study of the same reaction in similar green solvents. Collision-controlled reaction theory has been invoked to understand the role of encounter complexes to accelerate such a reaction at the molecular level in such highly viscous supersaturated solvent. No detrimental effects from the high viscosity of the new solvent medium on the reaction kinetics was been noted.

 Please wait while we load your content...
Please wait while we load your content...