Abstract
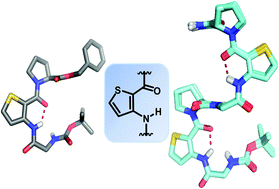
This paper describes the consequences of incorporating a constrained heterocyclic aromatic β-amino acid 3-aminothiophenecarboxylic acid (3-Atc) into peptides containing β-turn forming elements such as Pro-Gly motif and the effect on the secondary structural architecture of the entire peptide backbone. Conformational investigations of oligomers comprising an α,β,α peptide sequence were carried out by single-crystal X-ray diffraction, solution-state NMR, nOe-restrained MD simulation and circular dichroism studies. The results suggested that these peptide sequences assume helical architecture. The helical folding in the oligomers was found to be devoid of inter-residual H-bonding, instead found to be stabilized by a co-operative effect of 6-membered H-bonding within the 3-Atc unit and conformational restrictions of individual amino acids in the peptide backbone.

 Please wait while we load your content...
Please wait while we load your content...