Concise synthesis of a new triterpenoid saponin from the roots of Gypsophila oldhamiana and its derivatives as α-glucosidase inhibitors
Abstract
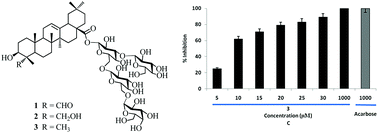
The first synthesis of the triterpenoid saponin 1 and its derivatives 2–3 was efficiently achieved in an efficient and practical strategy with an odourless 2-methyl-5-tert-butylphenyl (Mbp) thioglycoside and a N-phenyltrifluoroacetimidate donor as a key step, and their inhibitory activities against α-glucosidase and α-amylase were evaluated in vitro. The preliminary structure–activity relationship studies demonstrated that C4–CHO and C4–CH2OH were not essential to α-glucosidase inhibitory activity. Among the three compounds, compound 3 exhibited remarkably potent inhibitory activity against α-glucosidase with an IC50 value of 9.17 μM. According to Ki determined at 9.35 μM from the intercept on a Dixon plot, the more potent saponin 3 against α-glucosidase may be a noncompetitive inhibition pattern. In parallel, the lipophilicity of compounds 1–3 was calculated as a prediction for pharmacological potency. According to the predicted log P values, lipophilicity may correlate with the evaluated biological potency.

 Please wait while we load your content...
Please wait while we load your content...