Barbituric acid–triphenylamine adduct as an AIEE-type molecule and optical probe for mercury(ii)†
Abstract
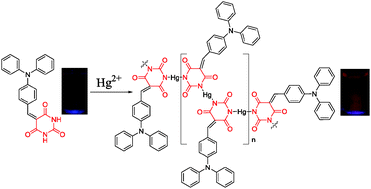
A type of simple-structured triphenylamine–barbituric acid adduct, M1, has been synthesized using a practical and simple Knoevenagel reaction under mild conditions. M1 displayed interesting aggregation-induced emission enhancement (AIEE) characteristics in a THF (good solvent)–water (poor solvent) system. The fluorescence intensity of M1 increased significantly when the water fraction in the co-solvent system surpassed 70%, and the emission maxima shifted from ∼568 nm (in THF) to ∼610 nm (in THF/water = 1 : 19, v/v). By taking advantage of the specific binding of the thymine-like amide groups in M1 with Hg2+, the fluorescence of M1 was enhanced to realize the successful turn-on detection of Hg2+ in an aqueous environment (THF/water = 3 : 7, v/v). Hg2+-induced aggregation of M1 is a plausible reason for the probing process.

 Please wait while we load your content...
Please wait while we load your content...