Thermosensitive antibacterial Ag nanocomposite hydrogels made by a one-step green synthesis strategy†
Abstract
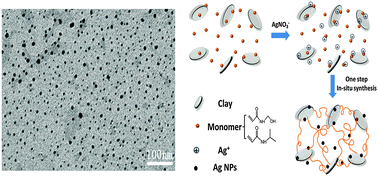
We developed a simple one-step synthesis route to prepare silver nanoparticles containing nanocomposite hydrogels by in situ free-radical copolymerization of N-isopropylacrylamide and N-(hydroxymethyl) acrylamide. In this synthesis, the hydrogels and nanoparticles are formed simultaneously without any additional reducing agent at room temperature, thus demonstrating the synergetic effect between reductive monomer HMAm and catalytic clay nanosheets in promoting the reduction of silver ions. The in situ generated silver nanoparticles within the hydrogel matrix were highly stable and the hydrogels showed sustained release of silver for periods of at least ten days in aqueous solution. Interestingly, the mechanical properties of the hydrogels showed remarkable improvement upon addition of a small amount of AgNPs. Furthermore, the resulting gels exhibited thermosensitive antibacterial activity, which was enhanced with an increase in the N-isopropylacrylamide content of the copolymers. Our work provides a facile and convenient green approach to preparing antibacterial tough hydrogels, which may find potential applications in wound dressings, tissue engineering and catalysis.

 Please wait while we load your content...
Please wait while we load your content...