Temperature-controlled Mukaiyama aldol reaction of cyclododecanone (CDD) with aromatic aldehydes promoted by TMSCl via the (TMS)3Si intermediate generated in situ†
Abstract
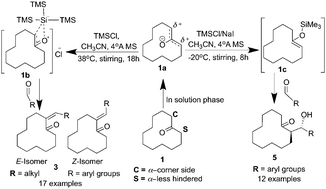
An alternative method with temperature dependency for obtaining direct chemo-, regio- and diastereoselective monobenzylidene and β-hydroxy carbonyl derivatives has been developed. In the present work, an attempt has been made to synthesize a precursor containing an organometallic compound, trimethylsilyl chloride (TMSCl), and cyclododecanone (CDD). Unexpectedly, this precursor exhibited temperature dependent chemoselective structures. At 35–40 °C, in situ formation of super silyl groups (TTMSS) from trimethylsilyl chloride stabilized the positive charge on the α-corner (C) side (sterically hindered side) of the CH2 group (1b) in zwitterionic CDD, leading to monobenzylidene derivatives (enones). At −20 °C, interestingly, TMSCl stabilized silyl enol ethers, which in turn produced β-hydroxy carbonyl derivatives (Mukaiyama aldol products) in the α-less hindered (S) side (sterically less hindered side) of the CH2 group. When we tried the reaction with TTMSSH instead of TMSCl, we failed to get either enones or aldol addition products. Tris(trimethylsilyl)silane (TTMSSH) stabilized the positive charge on the α-less hindered (S) side of the CH2 group. In the present protocol, the formation of monobenzylidene derivatives occurred in one step, whereas the methods available so far involved more than three steps. From this, it is clear that temperature is the only factor that changes the course of the reaction. In order to achieve diastereoselectivity in the Mukaiyama aldol reaction, sodium iodide was added. In monobenzylidene derivatives, the E-isomer is predominant (97–99%), while in the case of Mukaiyama aldol products, the anti-isomer is predominant (85–99%).

 Please wait while we load your content...
Please wait while we load your content...