Binuclear cyclooctatetraene–iron carbonyl complexes: examples of fluxionality and valence tautomerism†
Abstract
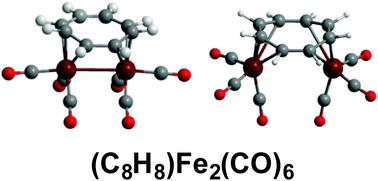
Reactions of cyclooctatetraene with iron carbonyls under various conditions give not only the monomeric (η4-C8H8)Fe(CO)3 but three C8H8Fe2(CO)6 isomers and one C8H8Fe2(CO)5 isomer. Density functional theory on the C8H8Fe2(CO)6 system shows the trans-(η4,η4-C8H8)Fe2(CO)6 isomer to be the lowest energy isomer. The cis-(η3,η3-C8H8)Fe2(CO)6 isomer with an Fe–Fe bond and an uncomplexed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the C8H8 ring lying ∼11 kcal mol−1 in energy above trans-(η4,η4-C8H8)Fe2(CO)6 appears to correspond to one of the metastable C8H8Fe2(CO)6 isomers obtained under relatively mild conditions. However, the cis-(η4,η4-C8H8)Fe2(CO)6 structure without an Fe–Fe bond suggested for the other metastable isomer appears to be a high-energy structure with a large imaginary vibrational frequency. Following the corresponding normal mode leads to cis-(η3,η3-C8H8)Fe2(CO)6. For C8H8Fe2(CO)5 the two lowest energy structures are singly bridged cis-C8H8Fe2(CO)4(μ-CO) structures differing only by a 22.5° rotation of the C8H8 ring around the central Fe2 unit. One of these structures is the experimental C8H8Fe2(CO)5 structure. The closeness in energy of these two C8H8Fe2(CO)5 structures is consistent with the experimentally observed fluxionality of this molecule in the NMR spectrum at low temperatures. The unsaturated C8H8Fe2(CO)n (n = 4, 3) structures obtained by further decarbonylation of C8H8Fe2(CO)5 retain the bridging bis(tetrahapto) or bis(pentahapto) C8H8 rings of C8H8Fe2(CO)5 and provide examples of structures with formal Fe
C double bond in the C8H8 ring lying ∼11 kcal mol−1 in energy above trans-(η4,η4-C8H8)Fe2(CO)6 appears to correspond to one of the metastable C8H8Fe2(CO)6 isomers obtained under relatively mild conditions. However, the cis-(η4,η4-C8H8)Fe2(CO)6 structure without an Fe–Fe bond suggested for the other metastable isomer appears to be a high-energy structure with a large imaginary vibrational frequency. Following the corresponding normal mode leads to cis-(η3,η3-C8H8)Fe2(CO)6. For C8H8Fe2(CO)5 the two lowest energy structures are singly bridged cis-C8H8Fe2(CO)4(μ-CO) structures differing only by a 22.5° rotation of the C8H8 ring around the central Fe2 unit. One of these structures is the experimental C8H8Fe2(CO)5 structure. The closeness in energy of these two C8H8Fe2(CO)5 structures is consistent with the experimentally observed fluxionality of this molecule in the NMR spectrum at low temperatures. The unsaturated C8H8Fe2(CO)n (n = 4, 3) structures obtained by further decarbonylation of C8H8Fe2(CO)5 retain the bridging bis(tetrahapto) or bis(pentahapto) C8H8 rings of C8H8Fe2(CO)5 and provide examples of structures with formal Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Fe double bonds with the lowest energy such structures having triplet rather than singlet spin states. Viable carbonyl-rich (η2,η2-C8H8)Fe2(CO)8 and (η4,η2-C8H8)Fe2(CO)7 structures represent possible intermediates in the formation of the various C8H8Fe2(CO)n (n = 6, 5) species from cyclooctatetraene and iron carbonyls.
Fe double bonds with the lowest energy such structures having triplet rather than singlet spin states. Viable carbonyl-rich (η2,η2-C8H8)Fe2(CO)8 and (η4,η2-C8H8)Fe2(CO)7 structures represent possible intermediates in the formation of the various C8H8Fe2(CO)n (n = 6, 5) species from cyclooctatetraene and iron carbonyls.

 Please wait while we load your content...
Please wait while we load your content...