Crystal growth of clathrate hydrates formed with methane + carbon dioxide mixed gas at the gas/liquid interface and in liquid water†
Abstract
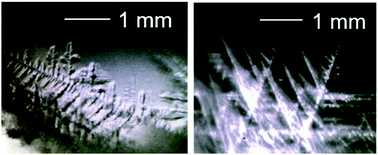
Visual observations of CH4 + CO2 hydrate crystal growth formed at the gas/liquid interface and in liquid water presaturated with a mixed gas have been made. The compositions of the CH4 + CO2 gaseous mixture were 40 : 60 and 30 : 70 for the gas/liquid interface observations, 30 : 70 and 70 : 30 for water saturated with the guest gas. The feed gas compositions of the CH4 and CO2 gaseous mixture were 40 : 60 and 30 : 70 for the gas/liquid interface observations, or 30 : 70 and 70 : 30 for liquid water. The crystal morphology of the CH4 + CO2 hydrate observed in both feed gas compositions was similar. This may be ascribed to the fact that the molar ratios of CO2 to CH4 in the liquid phase ranged from 90 : 10 to 97 : 3 due to the greater solubility of CO2 in water. These results suggest that the crystal morphology of the CH4 + CO2 hydrate may be controlled by the guest composition in the liquid phase, not by the feed gas composition. As the system subcooling increased, the shape of the hydrate crystals changed from polygons to sword-like or dendrites. The implications for the process design of the hydrate-based technologies are discussed based on the observations.

 Please wait while we load your content...
Please wait while we load your content...