The role of polarizability in determining metal ion affinities in polymer-supported reagents: monoprotic phosphates and the effect of hydrogen bonding†
Abstract
The rational design of sensors, solid phase extractants, and cross-linked polymers for environmental remediation requires a pairing of the electronic properties of a targeted metal ion with a given ligand. Polarizability is a key property and values for metal ions have been tabulated. It is now proposed to evaluate ligand polarizabilities by their affinities for Au(III) and Eu(III) as representative soft and hard metal ions. This report centers on: phosphonate diethyl ester (PDE); pentaerythritol diethyl phosphate (pPenta); diprotic phosphonic acid (DPA); phosphinic acid (PA); pentaerythritol monoethyl phosphate (pPentaM); glycerol monoethyl phosphate (pGlyM); and ethylene glycol monoethyl phosphate (pEG1M). Results show that hydrogen bonding is an additional variable that is superimposed on the affinities observed when ion exchange sites are introduced into ligands. PDE and pPenta are classified as soft ligands since they have a high affinity for Au(III) and a low affinity for Eu(III). DPA has a significant affinity for Au(III) and Eu(III) from less acidic solutions which indicates that the phosphoryl oxygen remains soft but intra- and inter-ligand hydrogen bonding cause a loss in both coordinating and ion exchange strength. Changing from a diprotic to a monoprotic acid decreases hydrogen bonding and increases metal ion affinities as indicated by results with PA, which has a high affinity for Au(III) via coordination to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Eu(III) via ion exchange at the P–OH. FTIR spectra support the change in hydrogen bonding. Monoprotic pPentaM is an alternative with decreased hydrogen bonding and it has high affinities for both Au(III) and Eu(III). The FTIR spectrum for pPentaM exchanged with Eu(III) confirms ion exchange by an hypsochromic shift of 25 cm−1 for the P–O band. The ability to tune metal ion affinities with hydrogen bonding is promising for the design of new ligands.
O and Eu(III) via ion exchange at the P–OH. FTIR spectra support the change in hydrogen bonding. Monoprotic pPentaM is an alternative with decreased hydrogen bonding and it has high affinities for both Au(III) and Eu(III). The FTIR spectrum for pPentaM exchanged with Eu(III) confirms ion exchange by an hypsochromic shift of 25 cm−1 for the P–O band. The ability to tune metal ion affinities with hydrogen bonding is promising for the design of new ligands.
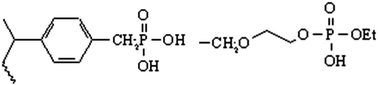

 Please wait while we load your content...
Please wait while we load your content...