New 3,4,5-trisubstituted isoxazole derivatives with potential biological properties†
Abstract
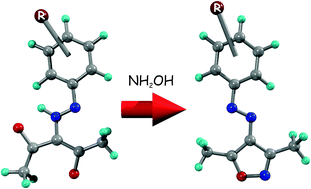
Synthesis of (E)-3,5-dimethyl-4-(R-phenyldiazenyl)isoxazoles was carried out by reacting β-diketohydrazones (R-C6H4-NHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(COCH3)2) with hydroxylammonium chloride, where R = 4-N(CH3)2(1), 4-OH(2), 4-CH3(3), 4-OCH3(4), 4-H(5), 4-Cl(6) 4-Br(7), 4-CO2H(8), 4-CO2CH2CH3(9), 4-COCH3(10), 4-CN(11), 4-NO2(12), 4-CH2CO2CH2CH3(13), 3-Cl(14), 2-OH(15), 2-Cl(16), 2-NO2(17), 2-CO2H(18). All compounds were characterized by EA and spectroscopic methods. The crystalline structure of two of the compounds, (7) and (17), were solved by the X-ray diffraction method. DFT and TDDFT computations were performed in order to characterize the molecular geometry and the molecular orbitals involved in the transitions. Besides, a biological activity study was performed to evaluate the toxicity of the compounds towards human promyelocytic leukemia cell line, HL-60, using the MTT reduction method. The IC50 values are in a wide concentration range, 86–755 μM. Isoxazoles (3) and (6) were the most cytotoxic. Expression analysis in HL-60 cells was carried out with compounds (3) and (6) by RT-PCR, in order to determine the effect on the expression levels of mRNA that codify for the genes Bcl-2, Bax and p21WAF-1. Isoxazole (3) induced a decrease in the expression of Bcl-2, whereas isoxazole (6) showed an opposite behaviour. However, these isoxazoles had no effect on mRNA levels of Bax. On the other hand, both isoxazoles increased the levels of p21WAF-1. These results suggest that the cytotoxic activity of isoxazole (3) would be the sum of effects triggered by promotion of apoptosis and cell cycle arrest, whereas for isoxazole (6) it would be mainly through cell cycle arrest.
C(COCH3)2) with hydroxylammonium chloride, where R = 4-N(CH3)2(1), 4-OH(2), 4-CH3(3), 4-OCH3(4), 4-H(5), 4-Cl(6) 4-Br(7), 4-CO2H(8), 4-CO2CH2CH3(9), 4-COCH3(10), 4-CN(11), 4-NO2(12), 4-CH2CO2CH2CH3(13), 3-Cl(14), 2-OH(15), 2-Cl(16), 2-NO2(17), 2-CO2H(18). All compounds were characterized by EA and spectroscopic methods. The crystalline structure of two of the compounds, (7) and (17), were solved by the X-ray diffraction method. DFT and TDDFT computations were performed in order to characterize the molecular geometry and the molecular orbitals involved in the transitions. Besides, a biological activity study was performed to evaluate the toxicity of the compounds towards human promyelocytic leukemia cell line, HL-60, using the MTT reduction method. The IC50 values are in a wide concentration range, 86–755 μM. Isoxazoles (3) and (6) were the most cytotoxic. Expression analysis in HL-60 cells was carried out with compounds (3) and (6) by RT-PCR, in order to determine the effect on the expression levels of mRNA that codify for the genes Bcl-2, Bax and p21WAF-1. Isoxazole (3) induced a decrease in the expression of Bcl-2, whereas isoxazole (6) showed an opposite behaviour. However, these isoxazoles had no effect on mRNA levels of Bax. On the other hand, both isoxazoles increased the levels of p21WAF-1. These results suggest that the cytotoxic activity of isoxazole (3) would be the sum of effects triggered by promotion of apoptosis and cell cycle arrest, whereas for isoxazole (6) it would be mainly through cell cycle arrest.

 Please wait while we load your content...
Please wait while we load your content...