Single and multiple peptide γ-turns: literature survey and recent progress
Abstract
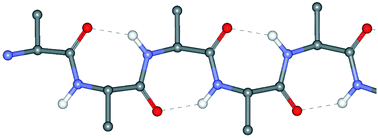
In contrast to the extensively investigated β-turn conformation in peptides and proteins, single and multiple γ-turns have been much less commonly studied. Single and non-contiguous multiple γ-turns have been relatively often authenticated in small cyclic peptides, but these important peptide main-chain reversal motifs have not been examined carefully either in linear peptides or in globular proteins. This Perspective article summarizes literature data on this aspect of peptide stereochemistry, expanding the discussion also to the rarely found, contiguous multiple γ-turns which generate incipient or fully-developed 2.27-(γ-) helices. Unpublished results of recent research activities on this topic ongoing in our laboratories are also briefly outlined.
- This article is part of the themed collection: Foldamer Chemistry

 Please wait while we load your content...
Please wait while we load your content...