The electrochemical synthesis of Pt particles on ZrO2–ERGO modified electrodes with high electrocatalytic performance for methanol oxidation†
Abstract
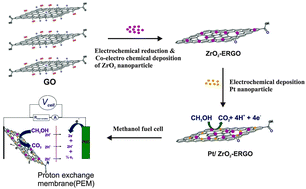
We report on a process for the electrochemical synthesis of Pt particles on a composite of zirconium oxide/electrochemically reduced graphene oxide (ERGO) sheets. Zirconium oxide produces bridging molecules which allow easy anchoring of Pt particles to form a functional ERGO multilayer film produced through a co-electrochemical deposition procedure. The catalytic performance improves as a consequence of the addition of ZrO2, which increases the number of active Pt sites. Scanning electron microscopy (SEM), Raman spectrometry, X-ray diffraction (XRD), and electrochemical impedance spectroscopy (EIS) are used to characterize the microstructure and morphology of the fabricated Pt/ZrO2–ERGO electrode. It is found that this approach allows for the development of new kinds of electrocatalysts for use in direct methanol fuel cells. The process of methanol oxidation is investigated through cyclic voltammetry and amperometry. The results indicate that the Pt/ZrO2–ERGO electrocatalyst exhibits much higher catalytic activity and better stability than either the Pt/ERGO or commercially available Pt/C electrocatalysts as well as have better tolerance to CO during the electrooxidation of methanol. The Pt catalysts on the ZrO2–ERGO composite facilitate the methanol oxidation reaction making it a promising material for application in the direct methanol fuel cells that are used in the fields of biotechnology and environmental chemistry.

 Please wait while we load your content...
Please wait while we load your content...