Zn4B3O9C2H8N2: an organic–inorganic hybrid borate with a novel graphene-like layer†
Abstract
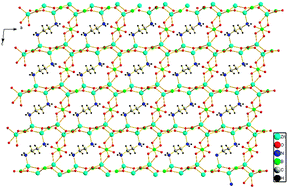
A new organic–inorganic hybrid zinc borate, Zn4B3O9C2H8N2, has been prepared under hydrothermal conditions, aiming at the formation of an extended coordination polymer with Zn(II) ions. It crystallizes into a monoclinic crystal system with a centrosymmetric space group of P21/c with a = 8.398(3) Å, b = 4.9346(18) Å, c = 14.205(4) Å, β = 95.64(2)°, and Z = 2. X-Ray diffraction analyses of available single crystals reveal novel graphene-like ZnBO layers which are connected by the disordered BO3 and ethylenediamine molecules. Formation of the corresponding zinc borate has been monitored and characterized by chemical and spectroscopic techniques. Characterization using powder X-ray diffraction, infrared spectroscopy, the UV-Vis diffuse reflectance spectrum, and the TG curve is also described.

 Please wait while we load your content...
Please wait while we load your content...