Synthesis, characterization, antibacterial, antioxidant, DNA binding and SAR study of a novel pyrazine moiety bearing 2-pyrazoline derivatives†
Abstract
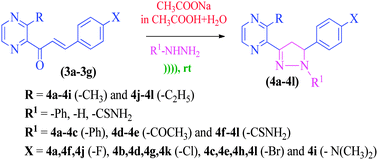
An environmentally safe method for the synthesis of novel substituted 2-(5-(4-aryl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-3-alkylpyrazine, 1-(5-(4-aryl)-3-(3-alkylpyrazin-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)ethanone and 5-(4-aryl)-3-(3-alkylpyrazin-2-yl)-4,5-dihydro-1H-pyrazole-1-carbothioamide via reactions of (E)-3-aryl-1-(3-alkyl-2-pyrazinyl)-2-propenones with phenyl hydrazine hydrochloride, hydrazine monohydrate and thiosemicarbazide respectively is reported. The reactions were carried out using sodium acetate–aqueous acetic acid solution at room temperature, under ultrasound irradiation (US). As compared to conventional methods, the advantages of the US method encompass milder conditions, operational simplicity, higher yield up to ≈93%, safety and environmentally friendly protocol. The synthesized compounds were evaluated in vitro for their antibacterial activities against Gram-positive and negative strains. Compounds 4a–4l showed 29–67% antioxidant activities, as determined through a 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical method. All synthesized compounds were screened for their DNA binding activities using a spectrofluorometer, where those containing halogens exhibited a significant potential (up to 570 ng mL−1) due to the corresponding intercalation and groove binding mechanisms.

 Please wait while we load your content...
Please wait while we load your content...