3,5-Bis(dithioacetal) meso-aryl BODIPYs: selective chemodosimeters for Hg(ii) ions†
Abstract
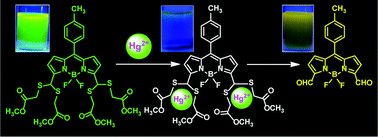
Boron-dipyrromethenes containing dithioacetal substituents at 3,5-positions, 3,5-bis(dithioacetal) BODIPYs 1 and 2, were synthesized by treating their corresponding 3,5-diformyl BODIPYs 3 and 4 with excess methyl thioglycolate under mild acid catalyzed reaction conditions. The spectral and electrochemical studies indicated that 3,5-bis(dithioacetal) BODIPYs are less electron deficient compared to 3,5-diformyl BODIPYs. Furthermore, dithioacetal functional groups are very useful for binding metal ions and our studies clearly showed that these dyes can act as selective chemodosimetric probes for the recognition of Hg(II) ions over various other metal ions. The sensing phenomenon employs unique and irreversible Hg(II) promoted deprotection of the dithioacetal groups to aldehyde groups which is clearly demonstrated by absorption, fluorescence, HR-MS and NMR studies. Competitive binding experiments demonstrate that BODIPY 1 can specifically detect Hg(II) ions even in the presence of various other metal ions.

 Please wait while we load your content...
Please wait while we load your content...