pH-dependent syntheses, luminescence and magnetic properties of two-dimensional framework lanthanide 1,3-diarylphosphonate complexes†
Abstract
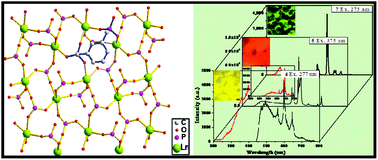
A series of novel organic–inorganic complexes based on trivalent lanthanides and 1,3-C6H4[CH2P(O)(OH)2]2 (H4L) formulated as Ln(HL)(H2O)2 (Ln = La, 1; Pr, 2; Nd, 3; Sm, 4; Eu, 5; Gd, 6; and Tb, 7) have been obtained as single phases by regulating the pH under hydrothermal conditions. Complexes are isostructural and feature a network in which the LnO7 polyhedra are interconnected by bridging CPO3 tetrahedra into inorganic layers. These layers are further stacked with the benzyl groups of ligands via C–H⋯π interaction into a laminar framework architecture. Luminescent measurements indicate that complexes 4, 5 and 7 show strong metal-centered emission bands in yellow, red and green light regions, respectively. Magnetic properties of 2 and 7 have also been studied.

 Please wait while we load your content...
Please wait while we load your content...